|
| [an error occurred while processing this directive] |
| Dan Little | Georgia Carroll | Jon Parrish |
| Pete Mikesell | James Gerken | Veronica Villalon |
| Richard Yee | Lisa LePome |
| Periodic Table |
Molecular Renderings |
Java Calendar |
*Puzzle |
Countries & Hits: [an error occurred while processing this directive]
|
Dr. Little received his Ph.D. in 1974 from the
University of Wisconsin, and was a postdoctoral fellow at Yale before joining
the faculty at UC Santa Barbara in 1975. He is the recipient of an A. P. Sloan
Foundation Fellowship and the H. J. Plous Award at UCSB. Dr. Little has been a
visiting professor at the University of British Columbia, served on the
Medicinal Chemistry Study Section of the NIH, and is currently Chair of the
Chemistry Department.
Our research focuses on the investigation and development of novel methodologies targeted for the production of biologically important molecules. Highlighted below are ongoing projects which involve the use of electroorganic synthesis and trimethylene methane (TMM) diradical chemistry. 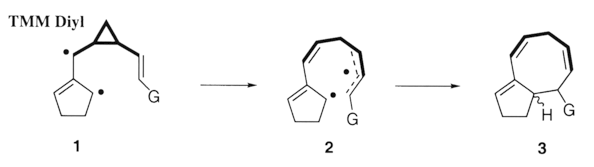
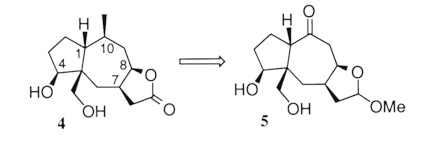
Elaboration of the substrate tether (emboldened in 4), has garnered us access to the [5.3.1] skeleton 6, the substructure prevalent in taxanes. This method is our current strategy in the syntheses of Taxol¨ analogs. 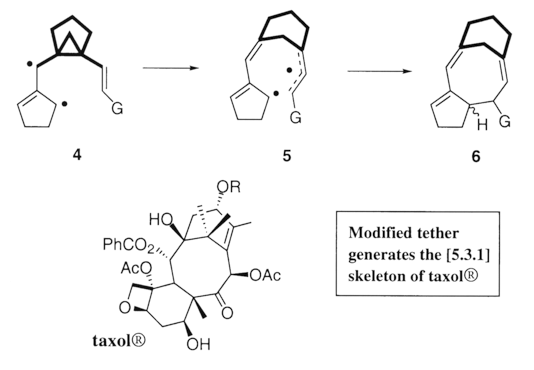
Electroreductive Cyclization; a Route to Phorbol Analogs 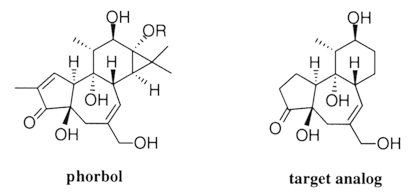 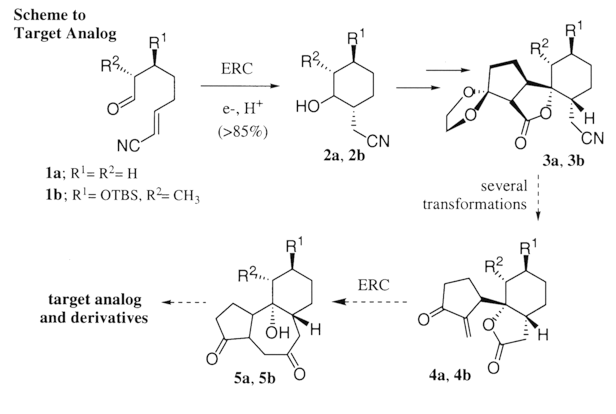
Cyclization experiments of model substrates have provided valuable insight towards the route we are now pursing.4 The ERC of both 1a and 1b routinely generate 2a and 2b (respectively) in 85-90%. Appendage of the cyclopentanone moiety was accomplished via intermolecular reductive coupling with SmI2. The stereochemistry of 3a was confirmed by x-ray crystallography. Investigation of the ERC reaction of substrate 4 is anticipated in the near future.
Atom Transfer of a TMM Diyl en route to Rudmollin 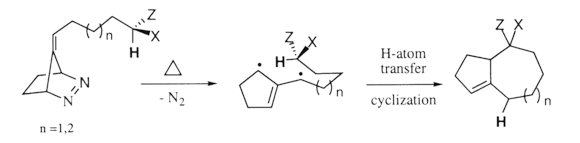
Rudmollin (1), our target of choice, is the major constituent of Rudbeckia mollis Ell., the common coneflower. This pseudoguaianolide exhibits in vivo activity against P-388 lymphoid leukemia.6 The structure and stereochemistry of 1 are well established,7 and only one total synthesis has been reported.8 Our approach is outlined in the retro-synthetic scheme, below. 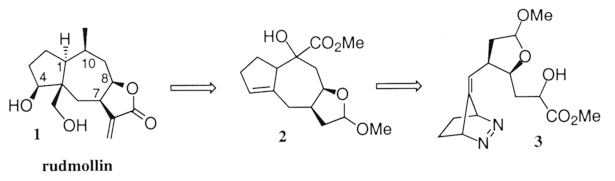
|
[an error occurred while processing this directive]